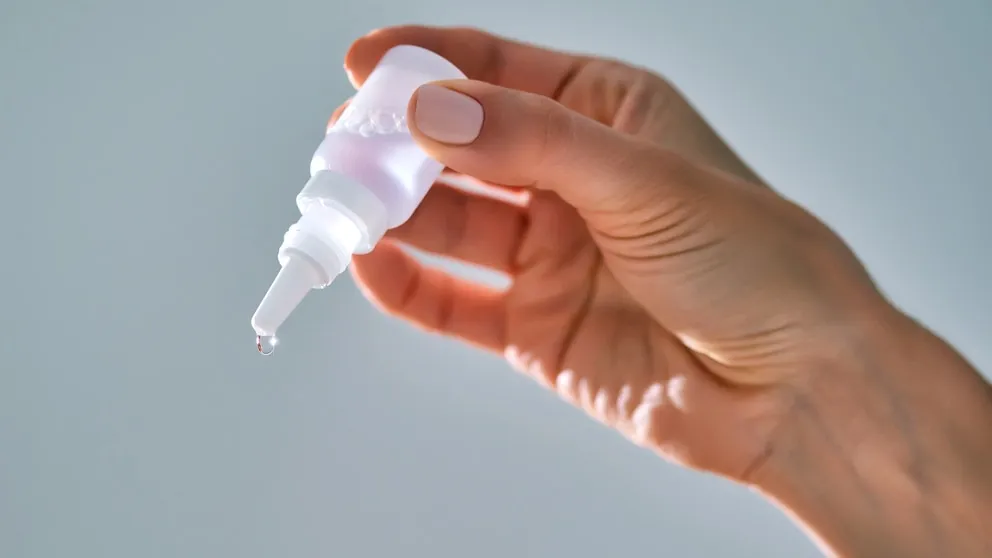
Nationwide Voluntary Recall: Dangerous Eye Drops and Eye Care Products Withdrawn from Shelves
In a recent announcement that has sent shockwaves through the healthcare community, a major manufacturer of eye drops has issued a voluntary recall of several artificial tears and other eye care products sold nationwide. This recall comes in response to mounting evidence of contamination and potential health risks associated with these products, sparking widespread concern among consumers.
Understanding the Recall
The recall, which affects numerous brands and product lines, has been prompted by reports of adverse reactions and contamination. The Food and Drug Administration (FDA) has reported an increase in complaints concerning these eye care products, which could lead to serious health complications for users. The company involved, whose name is not being disclosed due to ongoing investigations, has urged consumers to immediately discontinue the use of any affected products.
The Risk of Contamination
One of the primary concerns regarding the recalled eye drops is the potential for contamination with harmful bacteria. Specifically, the FDA has identified risks associated with Pseudomonas aeruginosa and other pathogens that can cause severe eye infections, particularly in individuals with weakened immune systems. Symptoms of such infections can range from mild discomfort to serious complications that may result in vision loss or systemic health issues.
How to Identify Recalled Products
Consumers are urged to check their medicine cabinets for the following products that are included in the recall:
- Artificial Tears – all sizes and brands associated with the manufacturer
- Eye lubricants – specific formulations that have been highlighted in the recall notice
- Other ophthalmic solutions that contain common preservatives
The affected products are typically sold across major retail chains, online marketplaces, and prescription outlets. Customers should review the packaging for batch numbers and expiration dates, which will indicate whether a product is part of the recall.
What Consumers Should Do
If you have purchased any of the recalled eye drops or eye products, it is critical to discontinue use immediately. The manufacturer recommends the following steps:
- Dispose of the affected product safely without causing harm to environmental sources, such as water supply.
- Monitor your health closely. If you experience any unusual symptoms such as redness, itchiness, or vision changes, seek medical attention promptly.
- Report any adverse reactions or health concerns to the FDA through their MedWatch Safety Information and Adverse Event Reporting program.
Expert Opinions on Eye Care Safety
Eye care specialists are voicing their opinions on the importance of vigilance regarding over-the-counter products. Dr. Jane Smith, an ophthalmologist based in New York, stated, “It’s crucial for consumers to be cautious when it comes to eye products. When you think about the sensitive nature of our eyes, any contamination can lead to significant health issues. Always check for product recalls and report anything suspicious.”
The Importance of Regulatory Oversight
The recent recall underscores the need for stringent regulatory oversight in the pharmaceutical and cosmetic industries. The FDA plays a pivotal role in ensuring the safety and efficacy of eye care products. However, the rise in recalls raises questions about current manufacturing practices and quality control measures.
In light of this incident, there are calls for enhanced regulations and a more diligent approach towards monitoring potential health hazards associated with consumer products. Consumers deserve transparency regarding the safety of items they use daily, especially products that directly impact their health.
Recalls Aren’t Uncommon
This recent recall is not an isolated incident. The FDA continually monitors the market for contaminated or hazardous products, leading to numerous recalls per year in the pharmaceuticals and personal care sectors. While recalls serve as an important safety net, they also highlight the essential nature of educating consumers about safe practices and awareness.
What’s Next for Consumers
As consumers, it is vital to stay informed about health and safety issues. One effective way to do this is to subscribe to alerts from the FDA and other relevant health organizations. Knowing what products could potentially pose a risk can help avoid serious health implications.
Moreover, seeking alternatives for eye care products should also be a priority. Opting for brands that prioritize transparency, quality checks, and consumer safety can lead to better outcomes for personal health. Always consult with healthcare providers before using new eye products, especially if you have pre-existing conditions or concerns.
Conclusion
The recent voluntary recall of eye drops and other eye care products reflects a significant public health concern that underscores the importance of product safety, regulatory oversight, and consumer vigilance. As the investigation into the affected products continues, consumers must act swiftly to ensure their health and well-being.
Additionally, it is crucial for the public to remain engaged, informed, and proactive about their eye health and skincare routines. The safety of our eyes depends not only on individual actions but also on collective awareness and robust industry standards.